NOAA practices advocacy science with major errors / lies about CO2 in official release
Carbon Dioxide at NOAA’s Mauna Loa Observatory reaches new milestone: Tops 400 ppm
May 10, 2013
Contact: John Ewald, 240-429-6127
On May 9, the daily mean concentration of carbon dioxide in the atmosphere of Mauna Loa, Hawaii, surpassed 400 parts per million (ppm) for the first time since measurements began in 1958. Independent measurements made by both NOAA and the Scripps Institution of Oceanography have been approaching this level during the past week. It marks an important milestone because Mauna Loa, as the oldest continuous carbon dioxide (CO2) measurement station in the world, is the primary global benchmark site for monitoring the increase of this potent heat trapping gas.nonsense
Carbon dioxide pumped into the atmosphere by fossil fuel burning and other human activities is the most significant greenhouse gas (GHG) contributing to climate change. Its concentration has increased every year since scientists started making measurements on the slopes of the Mauna Loa volcano more than five decades ago. The rate of increase has accelerated since the measurements started, from about 0.7 ppm per year in the late 1950s to 2.1 ppm per year during the last 10 years.
“That increase is not a surprise to scientists,” said NOAA senior scientist Pieter Tans, with the Global Monitoring Division of NOAA’s Earth System Research Laboratory in Boulder, Colo. “The evidence is conclusive that the strong growth of global CO2 emissions from the burning of coal, oil, and natural gas is driving the acceleration.”
Before the Industrial Revolution in the 19th century, global average CO2 was about 280 ppm. During the last 800,000 years, CO2 fluctuated between about 180 ppm during ice ages and 280 ppm during interglacial warm periods. Today’s rate of increase is more than 100 times faster than the increase that occurred when the last ice age ended.
NOAA’s Mauna Loa Observatory in Hawaii. Thursday, levels of the greenhouse gas carbon dioxide at Mauna Loa surpassed 400 parts per million for the first time since measurements began in 1958. Pre-industrial carbon dioxide levels were 280 parts per million.
It was researcher Charles David Keeling of the Scripps Institution of Oceanography, UC San Diego, who began measuring carbon dioxide at Mauna Loa in 1958, initiating now what is known as the “Keeling Curve.” His son, Ralph Keeling, also a geochemist at Scripps, has continued the Scripps measurement record since his father’s death in 2005.
“There’s no stopping CO2 from reaching 400 ppm,” said Ralph Keeling. “That’s now a done deal. But what happens from here on still matters to climate, and it’s still under our control. It mainly comes down to how much we continue to rely on fossil fuels for energy.”
NOAA scientists with the Global Monitoring Division have made around-the-clock measurements there since 1974. Having two programs independently measure the greenhouse gas provides confidence that the measurements are correct.
Moreover, similar increases of CO2 are seen all over the world by many international scientists. NOAA, for example, which runs a global, cooperative air sampling network, reported last year that all Arctic sites in its network reached 400 ppm for the first time. These high values were a prelude to what is now being observed at Mauna Loa, a site in the subtropics, this year. Sites in the Southern Hemisphere will follow during the next few years. The increase in the Northern Hemisphere is always a little ahead of the Southern Hemisphere because most of the emissions driving the CO2 increase take place in the north.
Once emitted, CO2 added to the atmosphere and oceans remains for thousands of years.more nonsense. Tom Segalstad has shown many papers found the lifetime is just 5-7 years. Thus, climate changes forced by CO2 depend primarily on cumulative emissions, making it progressively more and more difficult to avoid further substantial climate change. Direct chemical measurements (90,000) in Europe showed CO2 was higher in the 1940s and 1800s than it is currently.
See compilation of replies in Climate Depot Special Report “CO2 nears 400ppm. Relax it is not global warming end times but only a big yawn”
---------
In Defense of Carbon Dioxide
The demonized chemical compound is a boon to plant life and has little correlation with global temperature.
By HARRISON H. SCHMITT AND WILLIAM HAPPER
Of all of the world’s chemical compounds, none has a worse reputation than carbon dioxide. Thanks to the single-minded demonization of this natural and essential atmospheric gas by advocates of government control of energy production, the conventional wisdom about carbon dioxide is that it is a dangerous pollutant. That’s simply not the case. Contrary to what some would have us believe, increased carbon dioxide in the atmosphere will benefit the increasing population on the planet by increasing agricultural productivity.
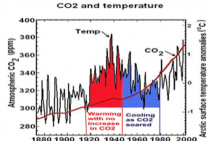
The cessation of observed global warming for the past decade or so has shown how exaggerated NASA’s and most other computer predictions of human-caused warming have been-and how little correlation warming has with concentrations of atmospheric carbon dioxide. As many scientists have pointed out, variations in global temperature correlate much better with solar activity and with complicated cycles of the oceans and atmosphere. There isn’t the slightest evidence that more carbon dioxide has caused more extreme weather.
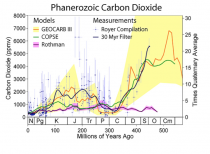
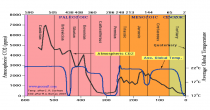
The current levels of carbon dioxide in the earth’s atmosphere, approaching 400 parts per million, are low by the standards of geological and plant evolutionary history. Levels were 3,000 ppm, or more, until the Paleogene period (beginning about 65 million years ago). For most plants, and for the animals and humans that use them, more carbon dioxide, far from being a “pollutant” in need of reduction, would be a benefit. This is already widely recognized by operators of commercial greenhouses, who artificially increase the carbon dioxide levels to 1,000 ppm or more to improve the growth and quality of their plants.
Using energy from sunlight - together with the catalytic action of an ancient enzyme called rubisco, the most abundant protein on earth - plants convert carbon dioxide from the air into carbohydrates and other useful molecules. Rubisco catalyzes the attachment of a carbon-dioxide molecule to another five-carbon molecule to make two three-carbon molecules, which are subsequently converted into carbohydrates. (Since the useful product from the carbon dioxide capture consists of three-carbon molecules, plants that use this simple process are called C3 plants.) C3 plants, such as wheat, rice, soybeans, cotton and many forage crops, evolved when there was much more carbon dioxide in the atmosphere than today. So these agricultural staples are actually undernourished in carbon dioxide relative to their original design.

Corbis
At the current low levels of atmospheric carbon dioxide, rubisco in C3 plants can be fooled into substituting oxygen molecules for carbon-dioxide molecules. But this substitution reduces the efficiency of photosynthesis, especially at high temperatures. To get around the problem, a small number of plants have evolved a way to enrich the carbon-dioxide concentration around the rubisco enzyme, and to suppress the oxygen concentration. Called C4 plants because they utilize a molecule with four carbons, plants that use this evolutionary trick include sugar cane, corn and other tropical plants.
Although C4 plants evolved to cope with low levels of carbon dioxide, the workaround comes at a price, since it takes additional chemical energy. With high levels of carbon dioxide in the atmosphere, C4 plants are not as productive as C3 plants, which do not have the overhead costs of the carbon-dioxide enrichment system.
That’s hardly all that goes into making the case for the benefits of carbon dioxide. Right now, at our current low levels of carbon dioxide, plants are paying a heavy price in water usage. Whether plants are C3 or C4, the way they get carbon dioxide from the air is the same: The plant leaves have little holes, or stomata, through which carbon dioxide molecules can diffuse into the moist interior for use in the plant’s photosynthetic cycles.
The density of water molecules within the leaf is typically 60 times greater than the density of carbon dioxide in the air, and the diffusion rate of the water molecule is greater than that of the carbon dioxide molecule.
So depending on the relative humidity and temperature, 100 or more water molecules diffuse out of the leaf for every molecule of carbon dioxide that diffuses in. And not every carbon dioxide molecule that diffuses into a leaf gets incorporated into a carbohydrate. As a result, plants require many hundreds of grams of water to produce one gram of plant biomass, largely carbohydrate.
Driven by the need to conserve water, plants produce fewer stomata openings in their leaves when there is more carbon dioxide in the air. This decreases the amount of water that the plant is forced to transpire and allows the plant to withstand dry conditions better.
Crop yields in recent dry years were less affected by drought than crops of the dust bowl droughts of the 1930s, when there was less carbon dioxide. Nowadays, in an age of rising population and scarcities of food and water in some regions, it’s a wonder that humanitarians aren’t clamoring for more atmospheric carbon dioxide. Instead, some are denouncing it.
We know that carbon dioxide has been a much larger fraction of the earth’s atmosphere than it is today, and the geological record shows that life flourished on land and in the oceans during those times. The incredible list of supposed horrors that increasing carbon dioxide will bring the world is pure belief disguised as science.
A version of this appeared in the WSJ. Mr. Schmitt, an adjunct professor of engineering at the University of Wisconsin-Madison, was an Apollo 17 astronaut and a former U.S. senator from New Mexico. Mr. Happer is a professor of physics at Princeton University and a former director of the office of energy research at the U.S. Department of Energy.
----------
The once great New York Times in denial on Benghazi is also carrying the water for the liar enviros and psuedo scientists with Justin Gillis’s continuing series of liar stories on CO2 impact here. Justin claims CO2 is the highest it has even been in the history of the earth. As you can see numerous studies have shown we are near the LOWEST levels in history. We need to push for higher CO2 to maximize crop production and drought resistance, and minimize water needs.
It also shows no convincing evidence of being associated with temperature changes.
Please bring back Andy Revkin, Gillis is clueless and NYT go back to covering ‘all the news thats fit to print’ accurately and in an unbiased way or you deserve to fail. And NOAA get back to covering weather and stop your advocacy science. Management nonsense is why employees on the front line gave management in NWS such low grades in survey.